Which Equation Describes A Reduction
Which equation describes a reduction? A- Upper M g (south) right arrow Uper Chiliad thousand superscript 2 plus (a q) plus two e superscript minus. B- 2 upper C fifty plus 2 east superscript minus correct arrow 2 upper C l superscript minus. C- Upper N a (s) right arrow upper N a superscript plus (a q) plus e superscript minus. D- Upper A fifty (south) right pointer upper A 50 superscript 3 plus (a q) plus 3 e superscript minus.
Related Question
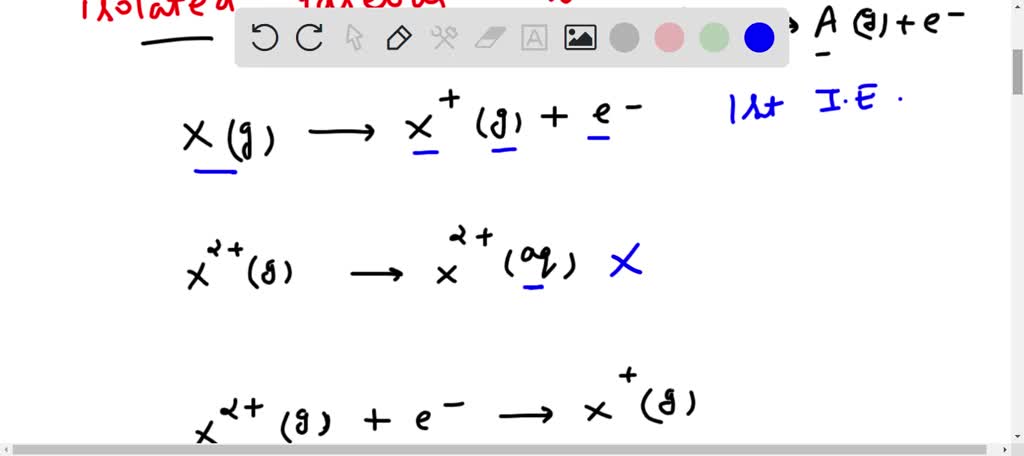
Which one of the following equations correctly represents the process involved in the 2nd ionization of X?a. X(g) --> Ten+(grand) + e-b. X+2 (g) --> X+2(aq)c. X2+ (g) + e- --> Ten+ (g)d. 10+ (thou) --> X2+ (g) + e-due east. 10+2 (thou) + Y2- (1000) --> XY (s)
Discussion
You must exist signed in to hash out.
Video Transcript
everyone. So in this question which one of the following situations right? Representative procedure involved in the second ionization of 10. So the concept in this question we use is the ionization energy. And according to the definition of ionization energy, it is the minimum amount of energy required to remove most loosely held electron from an isolated gaseous cantlet. That is known as ionization free energy. For example, if we have an atom A in the gaseous grade. And if you want to remove most closely held elections from the so you lot can meet how much free energy required to remove the most closely held electron from an isolated gaseous atom. That means the cantlet must be in free form. Information technology is not bound with any other cantlet like in gaseous form that is present in gratis form. Then how much energy required to remove that detail electron to class write a positive that is captain. So permit us see the equations given. So hither we have Ten in gaseous form, 10 positive in gashes plus electrons. So this is basically the first ionization free energy and in this case basically in the production side we have in Equus form. So that is not possible because information technology is defined only for the caches class, go your states and and so in this case Ten 2 positive plus electron. So you add the electron in X 2 positive. So this is also not the case. And the adjacent cases X positive in gaseous class to form Ten two positive gashes plus electron. So definitely this is the process in which the second ionization energy we can say that is involved. And in this instance that is also not possible because the solid face, it is not defined the ionization energy. So therefore based on that nosotros can say our correct respond is given in optionally the reason behind that. Run into if you have and atom X in gaseous class and yous want to remove an electron from this gaseous form. So you lot can see exposed to gasses class less electron. Then this is the ionization energy of one. At present y'all want to remove this i electron from this X. Positive. And then you can evidence like this so that is X two positive indications grade plus election. And this is basically the 2d ionization free energy. So therefore this equation that represent the second ionization free energy or the second ionization of X. So therefore the correct answer is given in option so therefore the correct answer is our pick. Did so I promise this answers your question.
Which Equation Describes A Reduction,
Source: https://www.numerade.com/ask/question/which-equation-describes-a-reduction-a-upper-m-g-s-right-arrow-uper-m-g-superscript-2-plus-a-q-plus-2-e-superscript-minus-b-2-upper-c-l-plus-2-e-superscript-minus-right-arrow-2-upper-c-l-sup-15482/
Posted by: paintersonch1974.blogspot.com



0 Response to "Which Equation Describes A Reduction"
Post a Comment